Introduction: Although current TKI options are often successful in managing CML-CP in early lines of therapy, patients may experience issues related to tolerability or resistance to TKIs. Physicians often have to proceed with treatment modifications including discontinuation, switching, temporary interruptions, or dose modifications when the patient's current treatment is not working as expected. This retrospective cohort study assessed these TKI therapy management patterns in patients with CML in first-line (1L) or second-line (2L) treatment in the US.
Methods: A large administrative claims database of commercially insured patients, the OptumInsight Clinformatics database (January 2007-June 2022), was used to select adult patients with CML who received 1L TKI therapy (imatinib, dasatinib, nilotinib, or bosutinib; 1L cohort) and those who received 2L TKI therapy (imatinib, dasatinib, nilotinib, bosutinib, or ponatinib; 2L cohort), with treatment initiation (index date) on or after 2012. Patients were required to have ≥6 months of continuous health plan enrollment pre-index, with no hematopoietic stem cell transplantation (HSCT) or CML-related chemotherapy treatments for accelerated phase (AP) or blast crisis (BC) pre-index. Treatment modifications assessed post-index included 1) treatment discontinuation, defined as the earliest of HSCT/treatment switch (defined below)/initiation of a CML-related chemotherapy for AP or BC, 2) treatment switch defined as initiation of another TKI/omacetaxine mepesuccinate, 3) temporary treatment interruption defined as gap of ≥ 14 days followed by resuming the index TKI, and 4) dose reduction (as compared to the initial dose) were assessed. Time to first event post-index were conducted using Kaplan Meier analyses for each of these treatment modifications, in 1L and 2L cohorts, separately.
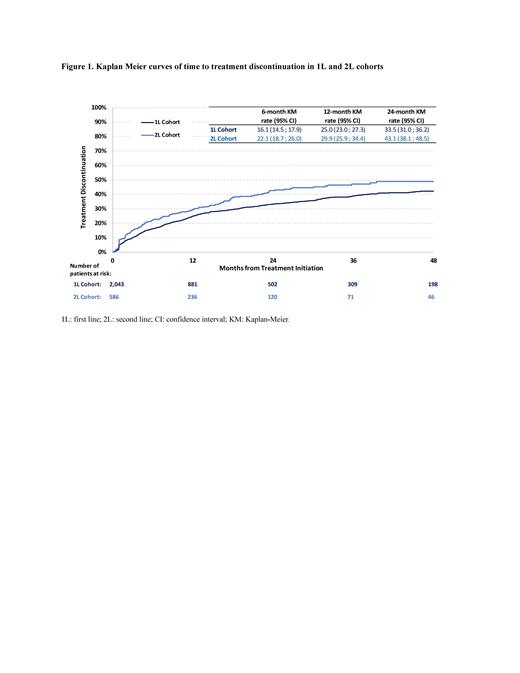
Results: A total of 2,043 patients were included in the 1L cohort with 47.8% of them receiving imatinib, 31.0% dasatinib, 19.2% nilotinib, and 2.0% bosutinib (median age: 64 years; 44.9% female; 69.7% White; 49.5% with Medicare advantage plan operated by commercial insurance [Part C]). Patients in the 1L cohort were observed for a mean (SD) period of 29.1 (27.3) months from index date to earliest of loss of follow-up/end of data. Overall, more than a quarter of patients (27.3%) experienced treatment discontinuation, with rates of discontinuation of 16.1% at 6 months, 25.0% at 12 months, and 33.5% at 24 months post-index ( Figure 1). Treatment switch was observed in 26.0% of the patients, with rates of 15.5% at 6 months and 24.4% at 12 months post-index. The proportion of patients with temporary treatment interruption was 18.6%, with rates of 20.6% at 6 months and 22.2% at 12 months post-index. Dose reduction (as compared to initial dose) occurred in 21.9% of patients, with rates of 18.8% at 6 months and 23.3% at 12 months post-index.
A total of 586 patients were included in the 2L cohort with 23.0% of them receiving imatinib, 36.9% dasatinib, 26.6% nilotinib, 10.9% bosutinib and 2.6% ponatinib (median age: 65 years; 47.3% female; 72.5% White; 48.3% with Medicare advantage plan operated by commercial insurance [Part C]). Patients in the 2L cohort were observed for a mean (SD) period of 28.4 (25.4) months from index date to earliest of loss of follow-up/end of data. Almost a third of patients (32.8%) experienced treatment discontinuation, with rates of discontinuation of 22.1% at 6 months, 29.9% at 12 months, and 43.1% at 24 months post-index ( Figure 1). Treatment switch was observed in 30.5% of patients, with rates of 20.5% at 6 months and 28.3% at 12 months post-index. The proportion of patients with temporary treatment interruption was 21.3%, with rates of 24.8% at 6 months and 25.4% at 12 months post-index. Dose reduction as compared to initial dose occurred in 20.6% of patients, with rates of 17.5% at 6 months and 27.2% at 12 months post-index.
Conclusions: Findings from this real-world study demonstrate that almost a quarter of patients with CML treated with current TKI treatment options in 1L or 2L experienced treatment discontinuation/switch in the first year from TKI initiation suggesting lack of optimal response/tolerability to currently available TKIs, with more pronounced rates in 2L. This suggests that unmet clinical needs still persist in this population, warranting optimal 1L and 2L treatment options with better tolerability profile for CML management.
Disclosures
Kota:Novartis: Honoraria; Kite: Honoraria; Pfizer: Honoraria; Incyte: Research Funding. Wei:Novartis: Current Employment, Current equity holder in publicly-traded company. Yang:Novartis: Current Employment. Romdhani:Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc., a consulting company which received funding from Novartis.; Analysis Group Inc: Current Employment, Other: I am an employee of Analysis Group, Inc., a consulting company which received funding from Novartis.. Latremouille-Viau:Bristol Myers Squibb: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Pfizer Inc.: Consultancy, Research Funding; Analysis Group, Inc.: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.. Guérin:AbbVie: Consultancy; Analysis Group: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.. Jadhav:Novartis: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal